Project 14: Chemical
Reactions Observations
Discussion:
Chemists
write Chemical Reactions using formulas and symbols to describe a chemical
change. These are the symbolic representation of a chemical sentence, consider
substance A and substance B undergoing a chemical change to produce substances
C and D, we write:

The
physical states of the chemicals are noted in the chemical equation by writing
the abbreviations (s), (l), (g), or (aq) for solid,
pure liquid, gas, or aqueous (water) solution.
Substances
A and
B
are called Reactants
or the subject of the sentence.
The
horizontal arrow reads “yields” or “produces” and is the verb of the sentence.
Substances
C
and D
are called Products
or the predicate of the sentence.
In CHM 1020 Suchocki text, watch video 9.1 and read
section 9.1:
Conceptual
Chemistry Chapter 9 Video Links
Chapter
9: How Chemicals React
9.1 Chemical
Reactions Are Represented
by Chemical Equations
Review the Chapter 9 Part I Study Pack-Sections A, A1, B, B1, D2, B3:
Chapter 9: Chemical Equations
A. Basic Reaction
Symbols-
Answers
A1. Classifying
Chemical Reactions- Answers
B.
Balancing
Chemical Equations Answers
B1.Predicting Single
Replacement Products Answers
B2.Predict Double
Replacement Answers
B3 Neutralization/Gas Forming Reactions Answers
Most textbooks* introduce the main types of simple inorganic chemical
reactions:
Types
of Chemical Reactions Hein
Corwin McMurry
|
Combination |
A
+ B à AB |
8.3 |
7.5 |
Not
introduced |
|
Decomposition |
AB
à
A + B |
8.3 |
7.6 |
Not
introduced |
|
Single
Replacement |
A
+ BC è B + AC |
8.3 |
7.8 |
Not
introduced |
|
Double
Replacement |
AB
+ CD à
AD + CB |
8.3 |
7.10 |
5.3,
5.4 |
*Suchocki does not introduce the types of chemical reactions; you
must use the study pack Chap 9 Part 1
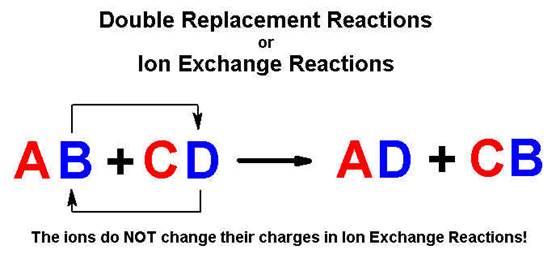
Ion
exchange reactions (precipitation and neutralization) are discussed in Parts D2
& D3 of Chapter 9 Part I Study Pack. Neutralization reactions (D2) are discussed
in Chapter 10 of CHM 1020 Suchocki Section 10.1. Please note the reactions show
in Table 10.1 page 300.
Single
Replacement Reactions:

E is a Metallic Element; A is also a Metallic Element or Hydrogen gas H2


Given the
following Partial
Activity Series:
Li > K > Ba > Sr > Ca > Na > (H2O) Mg > Al > Mn > Zn >
Fe > Cd > Co > Ni >Sn > Pb > (H)
> Cu > Ag > Hg > Au
(H) aqueous acid
Assignment:
For Combination and Decomposition Reactions watch the online chemical reactions and then record the observation of the chemical change. The balanced chemical equations are shown. For Single Replacement and Double Replacement Reactions watch the online chemical reaction, record your observations, then complete and balance the chemical reaction:
Chemical Reactions Experiment
Data Table/Pages:
A.
Combination Reactions
(Instructor Demonstration)
1.
Magnesium (s) + Oxygen (g) à
View Online: http://www.fccj.us/ReactionWithOxygen.mp4
Chemical Reaction D1: 2 Mg (s) +
O2 (g) à 2 MgO (s)
2.
Ammonia (g) + Hydrogen Chloride
(g) à
View Online: https://www.youtube.com/watch?v=Rf9j0ztzcs4
Chemical Reaction D2: NH3 (g) +
HCl (g) à NH4Cl (s)
3.
Sulfur (s) + Oxygen (g) (from Web) à
View Online: http://www.fccj.us/ReactionWithOxygen.mp4
Chemical Reaction D3: S (s)
+ O2 (g) à SO2 (g)
4.
Sodium (s) + Chlorine (g) (from Web)
à
View Online: http://www.fccj.us/SodiumChlorineCombination.mp4
Chemical Reaction: 2 Na (s) +
Cl2 (g) à 2 NaCl (s)
B.
Decomposition Reactions:
1.
Copper(II) sulfate penta Hydrate
à
View Online: https://www.youtube.com/watch?v=eX3JiKmenuU
Chemical Reaction #1: CuSO4∙5H2O (s) → CuSO4 (s) + 5 H2O (g)
2.
Cobalt(II) chloride hexa Hydrate
à
View Online: https://www.youtube.com/watch?v=-0a_zi0vhaE
Chemical Reaction #2: CoCl2∙6H2O (s) → CoCl2 (s) + 6 H2O (g)
3.
Nickel(II) chloride hexa Hydrate
à
View Online: https://en.wikipedia.org/wiki/Nickel(II)_chloride
Chemical Reaction #3: NiSO4∙6H2O
(s) → NiSO4 (s) + 6 H2O (g)
4.
Sodium hydrogen carbonate à
View Online: https://www.youtube.com/watch?v=OoPokz_5Sns
Chemical Reaction #4: 2 NaHCO3 (s) à Na2CO3 (s) + CO2 (g) + H2O (g)
C. Single Replacement Reactions:
1.
Calcium (s) + water (l) à
View Online: https://www.youtube.com/watch?v=i-rFsFwdkTU
Chemical Reaction #1: Ca (s)
+ HOH (l) à
2.
Magnesium (s) + water (l) à NR
Chemical Reaction #2: Mg (s)
+ H2O (aq) à NR
2`.
Magnesium (s) + Hydrochloric acid (aq) à
View Online: https://www.youtube.com/watch?v=SeKsLi_6WkY
Chemical Reaction #2`: Mg (s)
+ HCl (aq) à
3.
Copper (s) + Silver nitrate (aq) à
View Online: http://www.fccj.us/CopperSilverSingleReplacement.mp4
Chemical Reaction #3: (Copper becomes either +1 or +2 ion) Cu (s) + AgNO3 (aq) à
4.
Sodium (s) + water (l) à
View Online: http://www.fccj.us/SodiumPotassiumReactionWater.mp4
Chemical Reaction #4: Na (s) + HOH à
5.
Potassium (s) + water (l) à
View Online: http://www.fccj.us/SodiumPotassiumReactionWater.mp4
Chemical Reaction #5: K (s) + HOH à
6.
Zinc (s) + Tin(II) Nitrate (aq)
à
View Online: http://www.fccj.us/ZincTinSingleReplacement.mp4
Chemical Reaction #6: Zn (s) +
Sn(NO3)2 (aq) à
D. Double Replacement
Reactions (Precipitaion)
1.
Aluminum Chloride (aq) + Sodium carbonate (aq) à
View Online: https://socratic.org/questions/what-happens-when-you-mix-calcium-chloride-with-sodium-carbonate
Chemical Reaction #1: AlCl3(aq)
+ Na2CO3(aq) à
2.
Copper (II) sulfate (aq) + Sodium carbonate (aq) à
View Online: https://www.youtube.com/watch?v=VVRB0sV6Qq0
Chemical Reaction #2: CuSO4(aq)
+ Na2CO3(aq)à
3.
Silver (I) nitrate(aq) + Sodium carbonate (aq) à
View Online: https://www.youtube.com/watch?v=_lDLzmhF8E8
Chemical Reaction #3: AgNO3 (aq) +
Na2CO3(aq)à
4.
Aluminum nitrate(aq) + Sodium phosphate (aq) à
View Online: ???
Chemical Reaction #4: Al(NO3)3(aq) + Na3PO4(aq) à
5.
Copper (II) nitrate(aq) + Sodium phosphate (aq) à
View Online: ????
Chemical Reaction #5: Cu(NO3)2(aq) + Na3PO4(aq)à
6.
Silver nitrate(aq) + Sodium phosphate (aq) à
View Online: https://www.youtube.com/watch?v=HKbZhL8w84Q
Chemical Reaction #6: AgNO3 (aq) + Na3PO4(aq)à
7.
Copper(II) sulfate (aq) Sodium
bicarbonate (aq) à
View Online: https://www.youtube.com/watch?v=_xvut16Byxk
Chemical Reaction #7: CuSO4 (aq) + NaHCO3 (aq)à
8.
Cobalt(II) chloride (aq) Sodium
carbonate (aq) à
View Online: https://www.youtube.com/watch?v=lEdAkBNNqSY
Chemical Reaction #8: CoCl2 (aq) + Na2CO3
(aq) à
9.
Nickel(II) nitrate (aq) Sodium
carbonate (aq) à
View Online: https://www.youtube.com/watch?v=zqtXJS9tnRo
Chemical Reaction #9: Ni(NO3)2(aq) + Na2CO3 (aq) à
10.
Copper(II) chloride (aq) Sodium
phosphate (aq) à
View Online: https://www.youtube.com/watch?v=3thjr51qIN4
Chemical Reaction #10: CuCl2 (aq) + Na3PO4
(aq) à
11.
Cobalt(II) chloride (aq) Sodium
phosphate (aq) à
View Online: https://www.youtube.com/watch?v=i7satZ53G3Y
Chemical Reaction #11: CoSO4 (aq) + Na3PO4
(aq) à
12.
Nickel(II) chloride (aq) Sodium
phosphate (aq) à
View Online: https://en.wikipedia.org/wiki/Nickel_phosphate
(note color of product)
Chemical Reaction #12: NiSO4 (aq) + Na3PO4
(aq) à
E.
Double Replacement Reactions-Neutralization
1.
Nitric Acid (aq) Sodium Hydroxide (aq) à
View Online: https://www.youtube.com/watch?v=wnr-i_vA9nw
Chemical Reaction #1: HNO3(aq) + NaOH(aq) à
2.
Sulfuric Acid (aq) Sodium Hydroxide (aq) à
View Online: https://www.youtube.com/watch?v=8ZFfH3kfVBQ
Chemical Reaction #2: H2SO4(aq) +
NaOH(aq) à
3.
Phosphoric Acid (aq) Potassium Hydroxide (aq) à
View Online: https://www.youtube.com/watch?v=XKHxJeZTiy0
Chemical Reaction #3: H3PO4(aq) +
KOH(aq) à